Human factors engineering is a complex and multi-disciplinary area of risk management. It involves knowledge integration from various scientific fields, including psychology and engineering. The FDA plays a key role in regulating human factors engineering in medical devices in order to reduce the risk of injury to the user and the patient. The goal is to identify any errors that can result in serious harm before they happen and reduce or eliminate them.
What is human factors engineering?
In the United States, the FDA regulates human factors engineering in medical devices to reduce the risk of injury to the user and the patient. The FDA defines human factors engineering as:
“The application of knowledge about human behavior, abilities, limitations, and other characteristics of medical device users to the design of medical devices, including mechanical and software-driven user interfaces, systems, tasks, user documentation, and user training to enhance and demonstrate safe and effective use.”
In simple terms, human factors engineering uses the sciences of psychology and physiology to make products and devices based on their strengths and limitations. It is important in the medical industry because it keeps devices safe, effective, and comfortable. You can significantly reduce patient and user risk by incorporating human factors engineering into your design, labeling, instructions, and packaging.
How can you apply human factors to your medical device?
All medical devices need to have a certain level of usability and accessibility, so people will not be confused when they use them. As stated previously, proper application of human factors can decrease risk and maximize safety and efficacy. There are several ways that you can easily apply human factors engineering throughout the medical device design process.
First, start by identifying the intended users. You want to have an understanding of their abilities and limitations. Consider factors such as age, physical abilities, cognitive capabilities, and prior experience with similar devices. This knowledge will help you design with the user in mind.
When you begin designing, pay close attention to the user interface and strive for one that is intuitive, clear, and easy to navigate. Consider the arrangement of controls, use of icons, labeling, and organization of information. Additionally, consider ergonomic factors to ensure your device accommodates different body sizes, postures, and physical abilities. Design the device to be comfortable to hold, operate, and interact with for extended periods. For example, do not make a medical device that is too heavy or awkward to handle, as it can increase the risk of musculoskeletal injuries.
When it comes time to refine and test your prototype, conduct usability testing such as cognitive walkthroughs or heuristic evaluations. By doing so, you evaluate the device from the user’s perspective and confirm that it is easy to use, learn, and remember.
Finally, perform a thorough risk assessment to identify and mitigate potential use-related hazards. Consider factors such as user errors, misuse scenarios, and the consequences of those errors. Then, incorporate features or design considerations such as color and contrast, lighting, and auditory and visual warnings. You can also mitigate risks through thorough instructions, such as an instruction manual that can guide users through operating a device. The more details the instructions include, the more the risk factors will decrease. Since interpretations of products vary, clear instructions will help clarify device intent.

This cane grip has been ergonomically designed for maximum user comfort.
What human factors should you consider when designing your medical device?
Who is the user?
The characteristics of the intended users should be in your mind when designing your product. Ask yourself what kind of training the people handling it will have. If you are unsure, you should make the instructions clear enough, so anyone who may not have training can use your product. Users must know exactly how the product works to avoid injury or damage to the device. People often operate products that look like a previous one in the way the previous one worked. They will assume how a product works on prior knowledge, so directions must be detailed.
What is the environment?
When designing a product, the environment is an important factor to consider. Your product design and labeling must reflect the right conditions. For example, if the product is used in dim lighting, lighting features will need to be added to the design. If a product must be kept cool, that must be specified in the instructions and on the label.
How will the user interact?
Human factors engineers must take into account user interaction with the device. Every product, to decrease risk, requires clear instructions detailing how to use it and how it is supposed to function. All possible interactions need safety planning for anyone who could end up using the device. People will interact with the product by setting it up, cleaning it, packaging it, labeling it, and using it, so the design should incorporate and plan for all these interactions.
This part of the design process and the device user interface is the best area to make changes. For example, an easy place to eliminate hazards would include changing button placement or warning screens to better align with user habits. Unlike the environment and users, you can generally control your product and adjust it if needed.
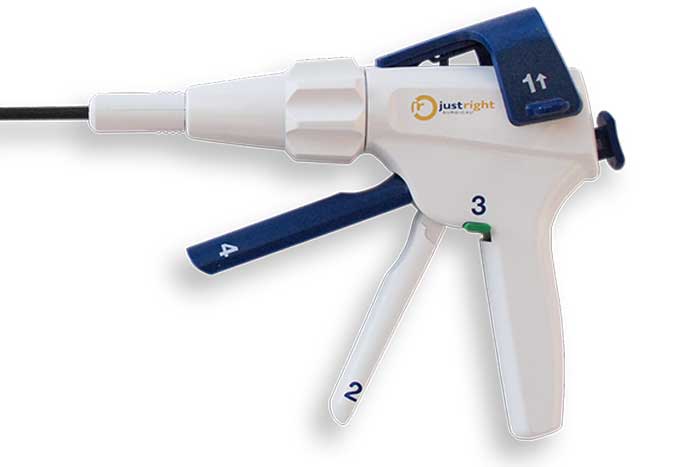
The handle of this medical device is labeled to indicate the order in which the device controls are to be used. Clear instructions on the device prevent misuse and use-related hazards.
How can you eliminate or reduce medical device use-related hazards?
The FDA guidelines outline risk management strategies for decreasing or eliminating use-related hazards of medical devices. Here are a few specific recommendations from the FDA for reducing use-related hazards in medical devices:
The first way is safety by design, which is when the design includes safety measures to make the use clear. Some examples include:
- Use standard symbols and conventions, such as “ON” and “OFF” labels for switches, to help users understand the device’s controls and indicators.
- Use appropriate user interfaces to be easy to use and understand and minimize the risk of errors.
- Design the device to be intuitive. The device’s controls and indicators should be easy to understand and use without extensive training or instruction.
The second way is through safety measures installed in the medical device, including:
- Use alerts for hazardous conditions that could put the patient at risk of severe injury or death, such as an emergency alert for a battery failure.
- Clearly label all controls, indicators, and displays in a way that is easy to read and understand.
- Use error-prevention and error-detection features.
Finally, include safety and training information, such as:
- Provide adequate training.
- Provide appropriate warnings and instructions to help users understand the associated risks.
- Provide instructions on properly maintaining and cleaning it.

The bright yellow label on this medical device warns anyone nearby that there is a risk of laser exposure.
How do you validate human factors?
Validating human factors occurs as the final test to test the safety and effectiveness of a product by assessing user-device interference that could result in patient or user harm. According to the FDA, human factors validation verifies the following:
- Test participants represent the intended users
- Critical device tasks are identified and tested
- The device-user interface is the final design
- The test conditions represent the current conditions
The validation of human factors involves testing the device with representative users to ensure that it is easy to use, learn, and remember, minimizing the risk of errors and injuries. Here are a few specific recommendations from the FDA for the validation of human factors in medical devices:
- Use various evaluation methods to validate the human factors of the device, including cognitive walkthroughs, heuristic evaluations, and user testing.
- Test with representative users to confirm that it is easy to use, understand, and minimizes the risk of errors and injuries.
- Test in the intended use environment to check that it is suitable for that environment.
- Use appropriate evaluation measures for the specific characteristics and intended device use.
- Thoroughly document the results, including any issues or problems identified and the actions taken to address them.
Every circumstance that could result in an error requires testing. The FDA requires testing on at least 15 United States citizens representative of the intended users in their natural environment. Different testing options for your product include user observation, interviews, and benchmark tests. If testing indicates that critical use errors occur, the FDA will not accept your device unless you can prove that harm reduction or elimination is no longer possible or the benefits of the products outweigh the damage.
How does the FDA regulate human factors engineering?
The FDA has established guidelines for human factors engineering in medical devices to ensure that the design minimizes the risk of errors and injuries. These include recommendations on usability testing, ergonomics, and other human factors considerations. Here are some specific guidelines that the FDA has issued for human factors engineering in medical devices:
- The device design should require use in a manner that minimizes the risk of errors and injuries.
- The device testing should be with representative users to ensure that it is easy to use, learn, and remember.
- The device should fit the user’s physical and cognitive abilities.
- The device’s user interface should minimize the risk of errors and maximize the user’s understanding of the device.
- The device’s color and contrast should enhance its usability and safety.
- The device’s lighting should maximize visibility and minimize glare.
- The device should have appropriate auditory and visual warnings to alert the user to potential risks.
These are just a few examples of the FDA guidelines for human factors engineering. Your medical device may require more or less, depending on its characteristics and intended use. Regardless of how many or few guidelines you follow, you must provide appropriate evidence that you have considered all relevant factors when submitting your medical device to the FDA for review.
In conclusion, incorporating human factors engineering into your medical device is essential for gaining FDA approval. Additionally, by optimizing the interaction between the device and the user, you can make a safe and effective device. By optimizing the interaction between the device and the user, human factors engineering helps to ensure device safety, effectiveness, and satisfaction. If you are unsure how to include human factors engineering in your medical device design or would like some expert advice while navigating the regulatory process, contact us, we would be happy to help.
Get a Free Quote
About Synectic Product Development: Synectic Product Development is an ISO 13485 certified, full-scale product development company. Vertically integrated within the Mack Group, our capabilities allow us to take your design from concept all the way to production. With over 40 years of experience in design, development, and manufacturing, we strive for ingenuity, cost-effectiveness, and aesthetics in our designs. Learn more about our medical device design services and see how we can help your next project.