A team of researchers at Rochester Institute of Technology has developed a new type of cardiovascular monitor in the form of a toilet seat. The seat, which aims to lower hospital readmissions for patients with congestive heart failure, would be issued to patients at discharge. The toilet seats are equipped with sensors that can monitor heart rate, blood pressure, blood oxygenation, stroke volume, and the patient’s weight. The data is analyzed by specialized algorithms and alerts providers if it detects the patient’s condition is deteriorating.
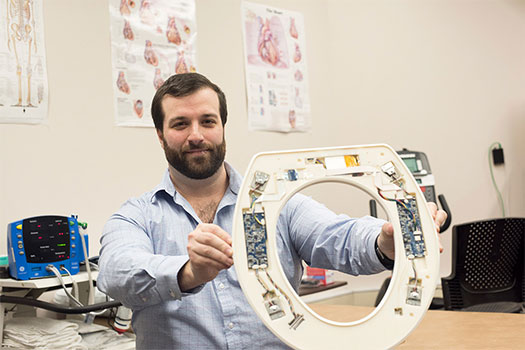
“Typically, within 30 days of hospital discharge, 25 percent of patients with congestive heart failure are readmitted,” says Nicholas Conn, a postdoctoral research fellow at Rochester Institute of Technology, who is part of the team developing the toilet seats. “After 90 days of hospital discharge, 45 percent of patients are readmitted. And the Centers for Medicare and Medicaid Services is penalizing hospitals for readmitting patients for heart failure.”
From the Rochester Institute of Technology article: “Conn, the company’s chief executive officer, further explains that using the national average for readmission rates, the penalty alone for readmitting 150 patients is approximately $500,000 annually. The total cost of providing 150 patients with their own monitored toilet seats from HHI is $200,000. With that investment, he says, hospital systems will save more than double their initial investment within one year.
According to Conn, who earned three degrees from RIT—a bachelor’s degree and a master’s degree in electrical engineering in 2011 and 2013, respectively, and a doctoral degree in microsystems engineering in 2016—the system will pick up deteriorating conditions before the patients even realize they are symptomatic. And with the rapid data analysis, interventions can be as simple as a drug change or short office visit, instead of an admission to the hospital.”
Read more about the toilet seat that detects congestive heart failure at Rochester Institute of Technology.
About Synectic Product Development: Synectic Product Development is a full-scale product development company. Vertically integrated within the Mack Group, our capabilities allow us to take your design from concept all the way to full-scale production. With over 40 years of experience in design, development, and manufacturing, we strive for ingenuity, cost-effectiveness, and aesthetics in our designs.