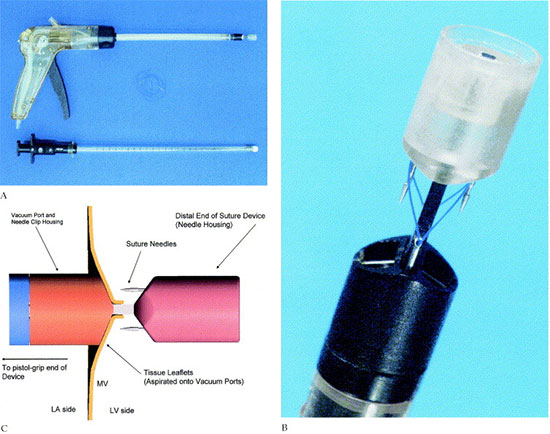
 Dr. Ottavio Alfieri’s paper on Novel Suture Device For Beating-Heart Mitral Leaflet Approximation details the successful use of Synectic developed mitral valve suture device in mitral valve repair on a beating heart without cardiopulmonary bypass. Read more about the study in The Annals of Thoracic Surgery.
Dr. Ottavio Alfieri’s paper on Novel Suture Device For Beating-Heart Mitral Leaflet Approximation details the successful use of Synectic developed mitral valve suture device in mitral valve repair on a beating heart without cardiopulmonary bypass. Read more about the study in The Annals of Thoracic Surgery.
About Synectic Product Development: Synectic Product Development is an ISO 13485 certified, full-scale product development company. Vertically integrated within the Mack Group, our capabilities allow us to take your design from concept to production. With over 40 years of experience in design, development, and manufacturing, we strive for ingenuity, cost-effectiveness, and aesthetics in our designs. Learn more about our medical device design services and see how we can help your next project.