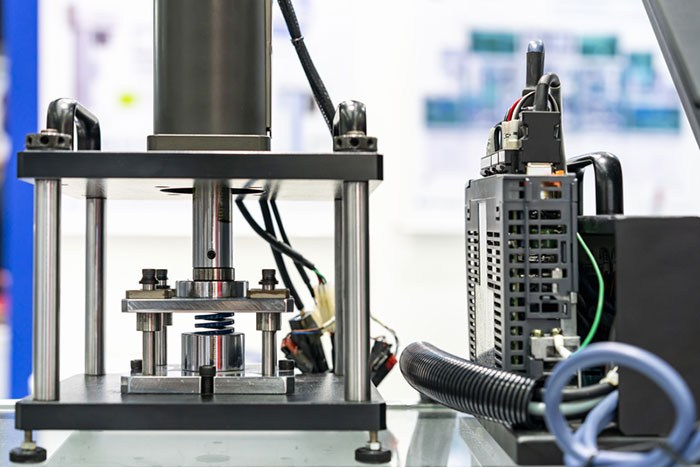
Prototype testing provides a cost-effective method for ensuring products meet user requirements and regulations. Simple proof of concept tests can predict future design problems and determine if further development is worth the effort. By starting this process in the early stages of prototype development, you minimize risk and save money. Further down the road, more targeted tests provide insights into the flaws of a prototype and guide design refinement. At the end of the engineering process, final prototype testing ensures that the product satisfies the product specifications and is safe for use. In short, prototype testing proves that your design is working or gives insight into why it failed. Here are several methods you can use to test your prototype to prove your invention works.
Breadboard testing
In the early stages of design, breadboards, or proof of concept prototypes are a cost-effective method of weighing the risks and benefits of different design concepts. These pre-alpha prototypes test the feasibility of an idea before developing it further. You would not want to get into tooling and manufacturing only to discover that your initial concept was never going to work. Breadboards are parts of systems you want to test, such as the human jaw or simple electronic circuits. The key to testing your breadboard is keeping it simple by making the most basic prototype possible. In some cases, testing can be as simple as seeing if the prototype fits in a particular space.
Computer simulation
Some computer-aided design (CAD) programs, such as Solidworks, virtually test CAD models using Finite Element Analysis (FEA) to predict the real-world behavior of the design. Computer simulation models test your design, exploring how parts and assemblies operate under stress. Knowing how parts respond to a load helps identify potential high-stress areas that may lead to design failure. Optimizing components without needing a physical prototype allows you to move quickly and cost-effectively through the early design process.
Force testing
Almost every product on the market will have some force applied to it at some point, making component durability is an essential test. You would not want a user to pick up your product and easily crush it. On the flip side, if your product requires a button, trigger, or lever, you need to confirm the user can apply enough force to activate it. Force testing finds the force necessary to operate a product and asserts the components of the product are strong enough to support those forces. The tests account for the maximum force a product can withstand and its lifespan under normal usage conditions.
Decibel testing
When working with fans, motors, or any audible device, decibel testing verifies that the device does not damage a patient’s auditory system. Additionally, if your device has an alarm or alert system that needs to be loud enough to be heard at a distance or under certain conditions, decibel testing proves that your device is functioning. Decibel testing uses sound level meters to find the noise level under various conditions. Then those measurements are compared with standard acceptable levels and any design or regulatory requirements. If the measurements are outside acceptable sound levels, design changes can be to the prototype before heading to production.
Airflow testing
If your product involves the flow of air, such as a fan or blower, airflow testing confirms that the proper amount of air is passing into, out of, and through your product. The test uses well-positioned airflow meters to measure airflow in various conditions and verify they meet product specifications and user requirements. Airflow testing also identifies design flaws or assembly problems that impede the flow of air. If your product contains cooling fans, the airflow path can give insight into maximizing cooling efficiency while minimizing product size.
Dielectric testing
When working with products that use electricity, dielectric testing, also known as hipot testing, confirms proper insulation of your product against voltage spikes during events such as a lightning strike. This test is also necessary to validate that there is no risk to the user of shock or electrocution upon contact with the device.
Dielectric testing is usually either a withstand or a strength test. With dielectric strength testing, the purpose is to determine at what voltage level the testing material fails. This failure happens when the test material experiences a sudden resistance change in response to the test voltage. The voltage that is measured when this occurs is the material’s dielectric strength. With dielectric withstand testing, you are testing the time it takes a material to fail at a specific high voltage. The time it takes for the material to experience a resistance shift is its dielectric withstand. In most cases, if your product is a medical device, dielectric strength testing is required for IEC-60601 and FDA approval.
Biocompatibility testing
Most medical devices require biocompatibility testing for FDA approval. Even if your product is not a medical device, you should seriously consider performing this test. For any product exposed to the human body for an extended period, this is especially true. In nonmedical products, such as wearable smart devices, this crucial test is often overlooked and can have devastating results both for your bottom line as well as your user. Even with a flawless design, a device may still cause damage to a patient if the materials become toxic through contamination, degradation, or leakage.
With wearable devices, prolonged exposure to certain materials may irritate the wearer leading to other serious complications. Biocompatibility testing guarantees the materials used in a product do not become toxic or cause irritation when exposed to the body. There are several ways to test for toxicity, ranging from chemical characterizations of degraded parts to extractable and leachable testing to biological testing. The level of testing required depends on the purpose and nature of the product. We recommend performing biocompatibility testing using an outside lab as it has the chemicals and procedures in place for proper testing.
Sterilization testing
This type of testing most often only applies to medical devices. Since these devices interact with the human body, they can be sources of infection if not properly cleaned. Sterilization testing assures the FDA that your device is delivered in a sterile condition and can be sterilized for reuse if necessary.
One method of sterilization testing is bioburden testing. In this test, the device is swabbed for micro-organisms to determine the total microbial count before the first sterilization and use. This test acts as an early warning system for possible production problems and contamination sources, leading to inadequate sterilization. Bioburden testing can also give insight into the necessary dose for effective radiation sterilization. Additionally, it demonstrates microbial quality control of the manufacturing process.
Packaging testing
Package testing is probably one of the most overlooked aspects of product development. After spending all this time and money developing the perfect product, it would be disastrous if you could not get it to your customer in one piece. Packages are tested using several methods such as force and drop testing to ensure devices reach their destination safely. It is vital to check that the packaging protects the device from both physical damage and chemical harm. If you use the wrong packaging material, it could create toxins when in contact with the product leading to harm to the user. If there is not enough material surrounding your product, shipping could damage it. Alternatively, if you use too much packing, the box may be too heavy or bulky to ship cost-effectively. A basic packaging test would involve shipping the packed product to yourself to check its condition upon arrival.
Package testing gets a little more complicated for sterile medical devices. For FDA approval, your product will require accelerated and real-time aging tests that demonstrate your package’s shelf life. These tests subject your packaged sterile device to elevated temperatures for a certain amount of time. The high temperature speeds up the aging process giving you the necessary data without having to wait years.
Some final thoughts
Before we wrap up, we need to touch upon a few points regarding prototype testing. While the specific types of tests covered above are product-specific, these points apply to all products.
- Make sure you calibrate your measurement devices. To get accurate test results, you need accurate measurements. Routine calibration of measurement tools is important, especially when used on a regulated product. We recommend calibrating your devices at least once a year or the calibration timeline outlined by the manufacturer.
- Make sure you have done enough testing. To check this, here is a simple exercise you can perform. Refer to your product development specification (PDS) and, for every specification, ask yourself if your prototype fulfills the requirement. If the answer is no, you either need to change the PDS to fit the design or change the design to conform with the PDS. If the answer is yes, then write down what test you have performed that proves this. If you cannot provide proof with a test result, then you need to do more testing.
- Make sure you have made enough prototypes to perform the necessary tests. Prototypes are broken, warped, ripped apart, and destroyed during testing. If any of these tests are for certifications, they may require that dozens of prototypes go through each test. If not, you should be collecting at least three data points, from three different devices, for each test. These prototypes also cannot be used in another test once already tested. At the very minimum, you should have one hundred prototypes specifically for testing.
In conclusion, you can never perform too much prototype testing. Test your product against misuse and human error. Test it upside-down, sideways, underwater, etc. Give it to your friends and family and observe how they use the product. Have children and the elderly use your product and see if they have any difficulty using it. The more testing you do, the better. Every test you perform and learn from will decrease your chance of having a financially crushing recall or a lawsuit if your product harms someone. You have put your heart and soul into this product, do not let testing be the one thing that ruins your success. If you need help writing your testing protocols we have put together a comprehensive editable Prototype Testing Workbook that will walk you step by step through the process. Download your free copy below!
Have You Tested Your Prototypes Enough?
We’ve put together a free prototype testing workbook for writing your protocols, setting up your tests, and building your reports.
Download your free workbook now!
"*" indicates required fields
About Synectic Product Development: Synectic Product Development is an ISO 13485 certified, full-scale product development company. Vertically integrated within the Mack Group, our capabilities allow us to take your design from concept all the way to production. With over 40 years of experience in design, development, and manufacturing, we strive for ingenuity, cost-effectiveness, and aesthetics in our designs. Learn more about our prototype development services and see how we can help your next project.




Comments are closed.