
Medical Device Design Controls Explained
Design controls are a set of practices and procedures incorporated into the design process as part of current good manufacturing practice (cGMP)

Design controls are a set of practices and procedures incorporated into the design process as part of current good manufacturing practice (cGMP)
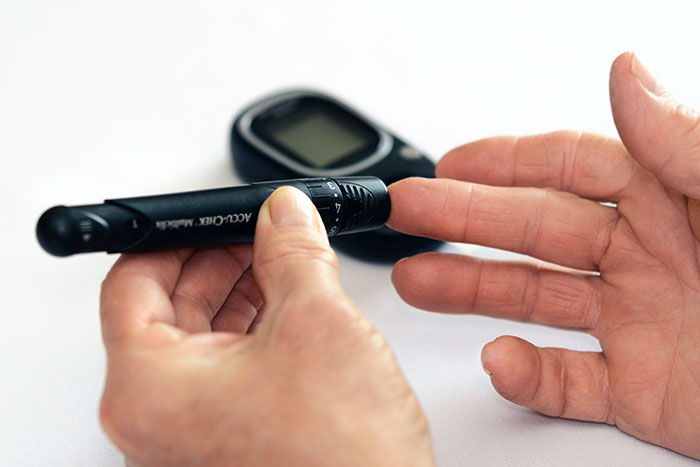
Regardless of what type of medical device you develop, the FDA requires you to follow a regulated process. Each step of medical
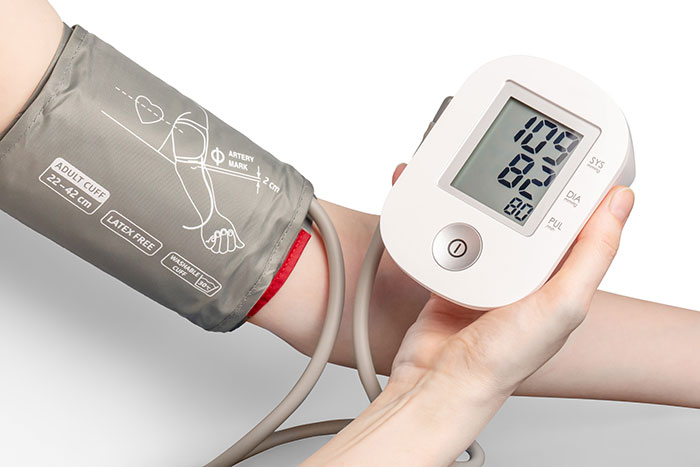
Human factors engineering is a complex and multi-disciplinary area of risk management. It involves knowledge integration from various scientific fields, including psychology

Verification and validation are design controls required by the FDA to ensure the medical devices you manufacture are safe, effective, and fit

Biocompatibility testing is the best way to evaluate medical device safety. Through biocompatibility testing, the FDA confirms that either there are none
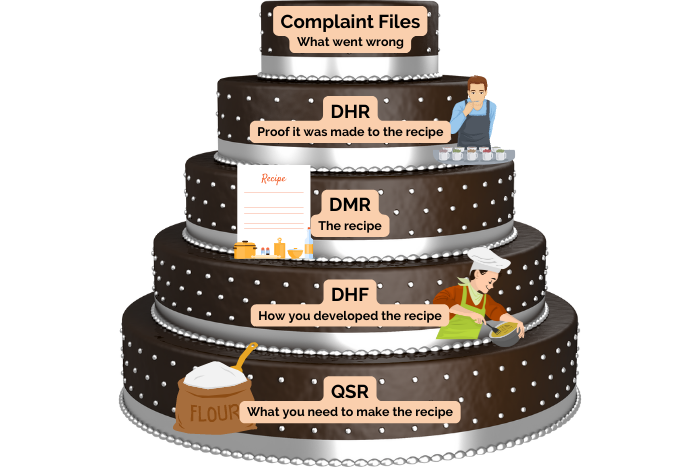
Listed within the FDA’s 21 CFR 820 are detailed regulations governing medical device records and quality systems. These regulations identify five record

21 CFR Part 820 is a set of FDA regulations that govern medical device quality systems. Detailed within are current good manufacturing practice

Part of the Medical Device Regulation Act involved establishing specific regulatory controls for each of the three classes of medical devices. The

In 1971 a new IUD called the Dalken Shield entered the market and was implanted in almost 3 million women in the

The challenge Fixtures are used in product development and manufacturing for a variety of purposes. Most commonly they are used as a
Notifications