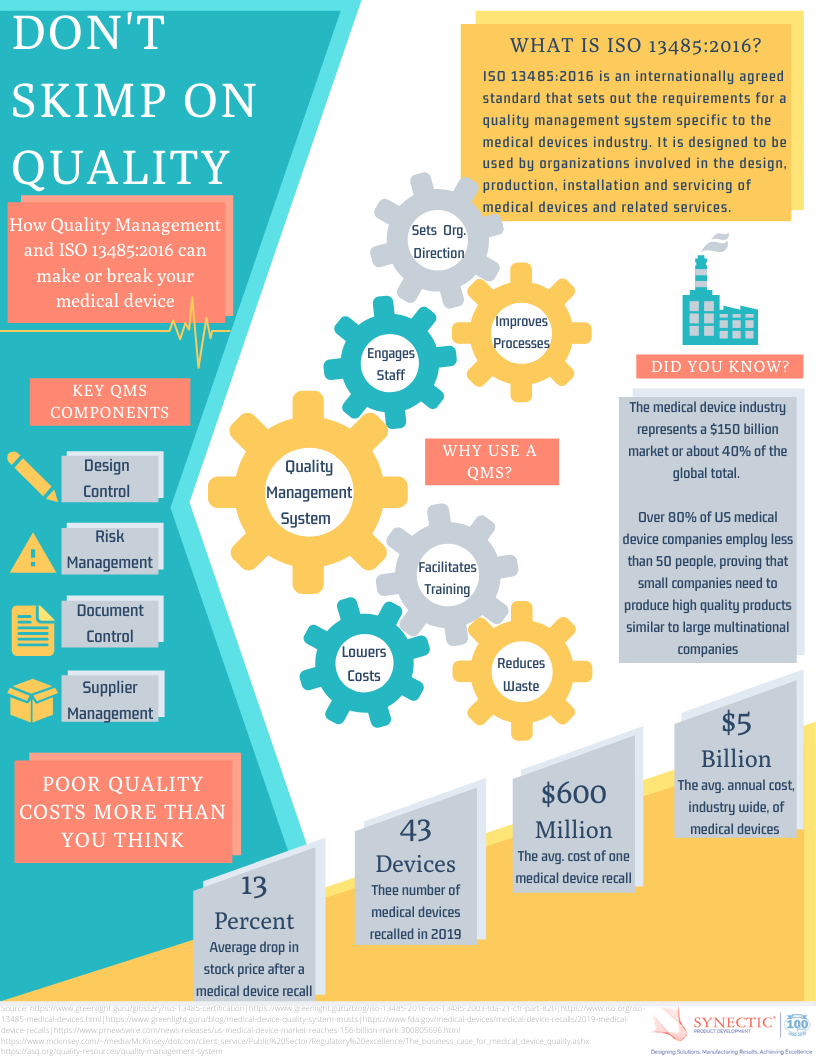
Need help developing an ISO compliant QMS?
Speak with one of our experts and get answers to all your project questions.
About Synectic Product Development: Synectic Product Development is an ISO 13485 certified, full-scale product development company. Vertically integrated within the Mack Group, our capabilities allow us to take your design from concept to production. With over 40 years of experience in design, development, and manufacturing, we strive for ingenuity, cost-effectiveness, and aesthetics in our designs. Learn more about our medical device design services and see how we can help your next project.
Recommended for you
Looking For Design Help?
Complete the form below to speak with our experts about bringing your medical device to market.