BLOOD CLEANSER
Extracoporeal Blood Cleansing Device
Sepsis is a life-threatening medical emergency caused by the body’s extreme response to infection. It occurs when an infection triggers widespread inflammation, leading to tissue damage, organ failure, and potentially death. According to the World Health Organization (WHO), sepsis affects over 49 million people annually and is responsible for approximately 11 million deaths worldwide, making it one of the leading causes of mortality. Currently, the only treatment option available for sepsis is antibiotics and supportive care. However, the condition is notoriously difficult to treat due to delayed diagnosis, antibiotic resistance, and rapid disease progression.
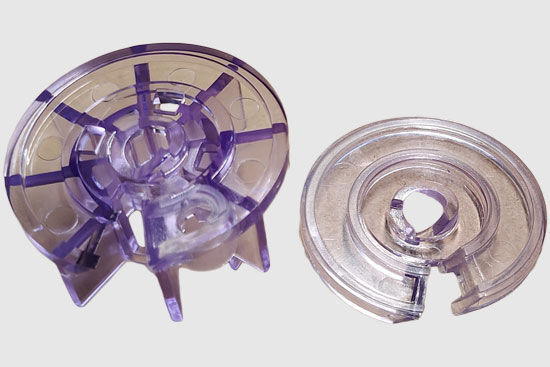
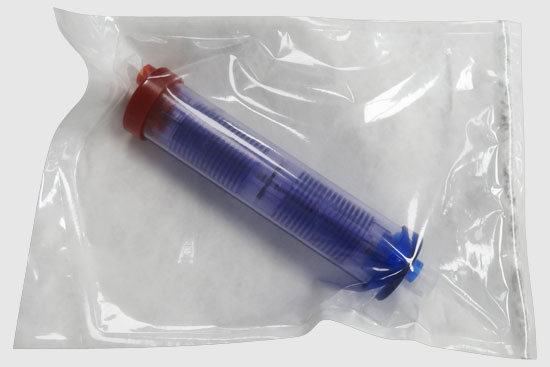
A blood filtration startup company contracted Synectic to develop a novel blood-cleansing device to treat sepsis. The device would directly capture and isolate bacteria and endotoxins in patients with sepsis. By physically removing pathogens and endotoxins from the bloodstream in real-time, the device would offer an immediate response to infection. The company came to us with a 2D proof of concept and tasked us with transforming it into a 3D design ready for production. Additionally, they required materials that could endure treatment with antibiotic solutions and withstand the high pressures generated by the dialysis pumps. We tackled these challenges through a systematic engineering and manufacturing approach where we:
- Conducted extensive research to identify materials compatible with centrifugal force and antibiotic coatings.
- Transformed the 2D flat design into a 3D cylindrical design that fits a standard dialysis pump.
- Developed an interlocking plate system that preserved the required geometry while maintaining robust pressure seals inside and out.
- Designed the device for manufacturability and FDA compliance, including Class III device requirements.
- Wrote and implemented rigorous testing protocols, including leak testing, subassembly leak testing, and occlusion testing, to ensure no epoxy contamination in the flow zone.
- Created innovative fixtures to streamline production, including a dual-purpose filter fixture and torque fixture for easy assembly.
- Prepared comprehensive quality documentation to meet FDA sterilization, verification, and validation standards.
- Addressed biocompatibility, aging, shipping, and packaging requirements to support Class III device classification and FDA approval.
The result was a functional, manufacturable, and FDA-compliant extracorporeal blood cleansing device. The device demonstrated over 95% reduction in bloodstream bacteria in trials in a single pass, marking a significant advancement in sepsis treatment.

SYNECTIC'S SOLUTION