MOST POPULAR WHITE PAPERS
ALL WHITE PAPERS

How to Design for 3D Printing
3D printing is a prototyping method that "prints" a three-dimensional physical object in layers from a digital file. Compared to traditional prototyping methods, 3D printing is fast and low-cost. While ... Read More

How to Perform a DFMEA In 9 Steps
FMEA stands for Failure Mode and Effects Analysis and is a risk management tool for identifying potential failures. FMEA assesses the severity of the failure risk and how to diminish ... Read More

How the FDA Defines and Evaluates Medical Device Biocompatibility
Biocompatibility testing is the best way to evaluate medical device safety. Through biocompatibility testing, the FDA confirms that either there are none or few local or systemic effects. Local effects ... Read More
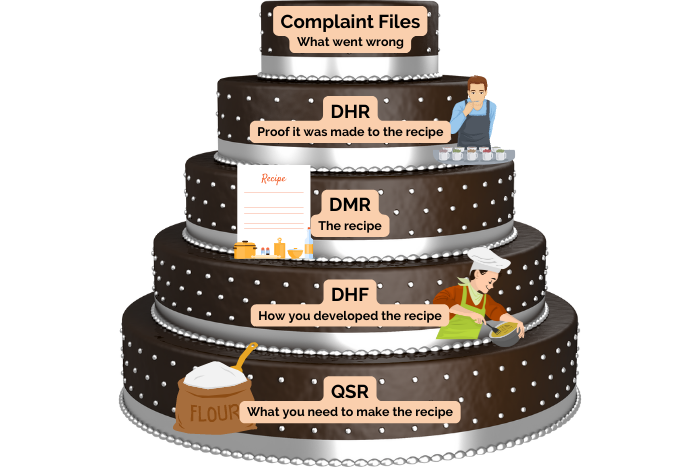
A Breakdown of the FDA’s Medical Device Record Requirements
Listed within the FDA’s 21 CFR 820 are detailed regulations governing medical device records and quality systems. These regulations identify five record types that medical device manufacturers need to maintain ... Read More

All About FDA 21 CFR Part 820 and cGMPs
21 CFR Part 820 is a set of FDA regulations that govern medical device quality systems. Detailed within are current good manufacturing practice (cGMP) requirements ensuring medical devices distributed within the ... Read More

FDA Medical Device Regulatory Controls Explained
Part of the Medical Device Regulation Act involved establishing specific regulatory controls for each of the three classes of medical devices. The higher the class of device, the more risk ... Read More

How the FDA Classifies Medical Devices
In 1971 a new IUD called the Dalken Shield entered the market and was implanted in almost 3 million women in the United States. Over the next few years, the ... Read More

4 Tips to Improve Your Pilot Production Build
The Pilot Production Phase is the beginning of the end of product development. Pilot Production is just a step away from contract manufacturing and is used at the end of ... Read More






